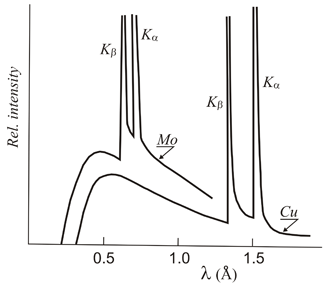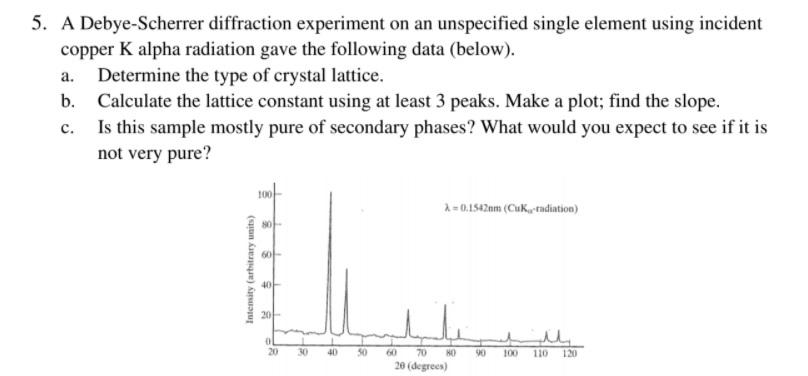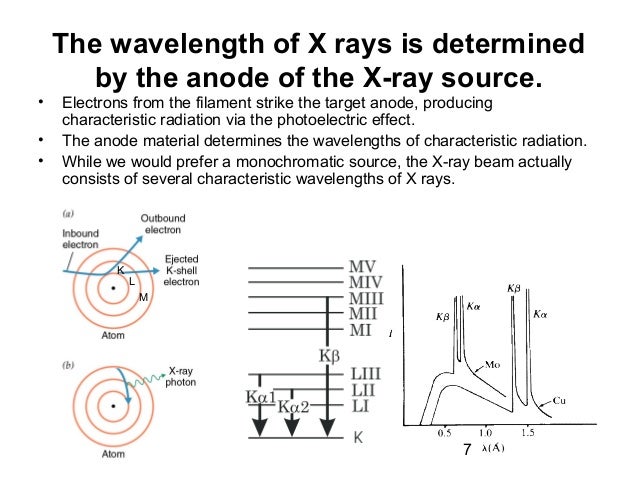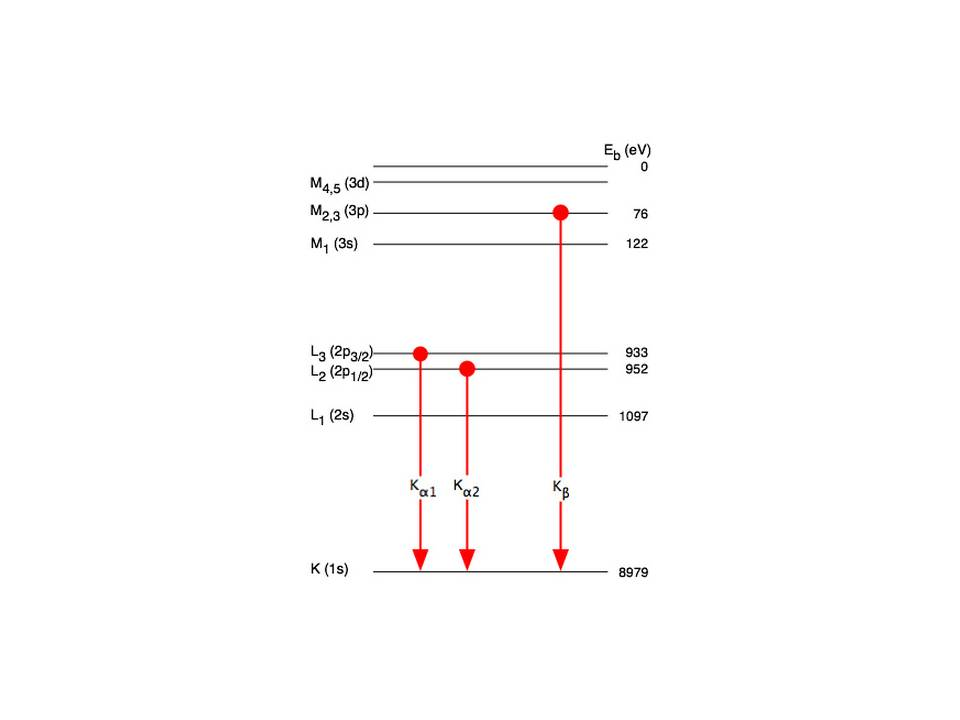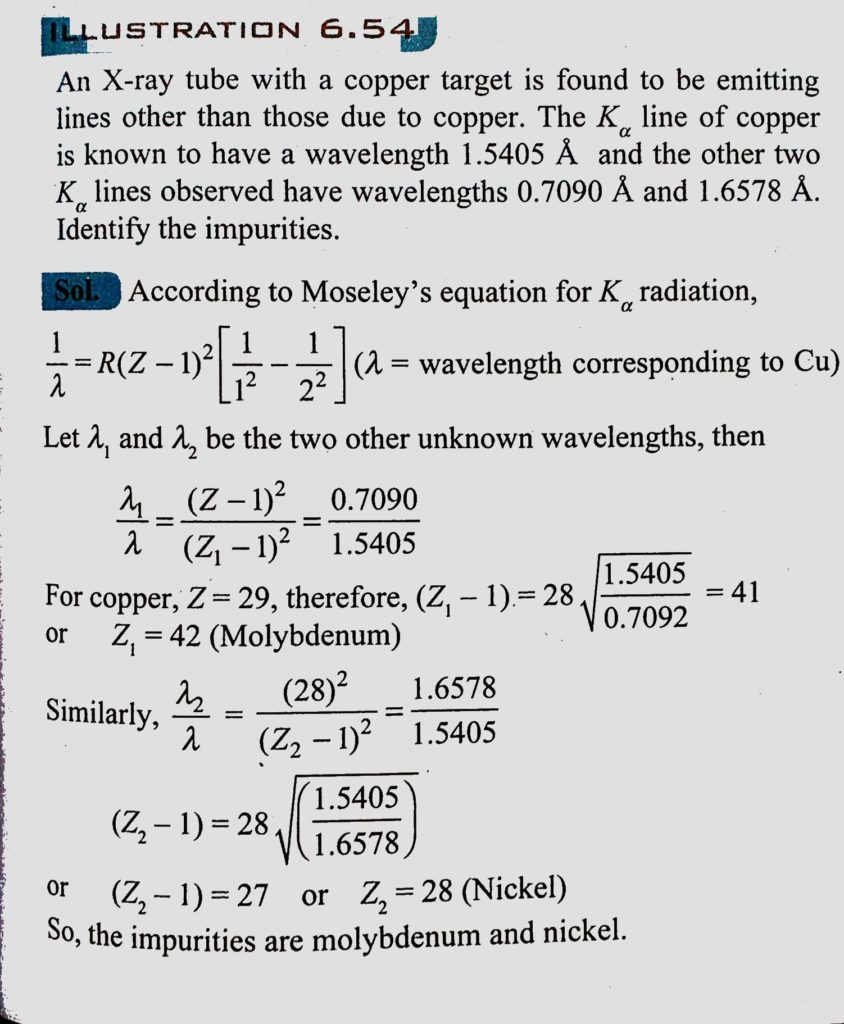
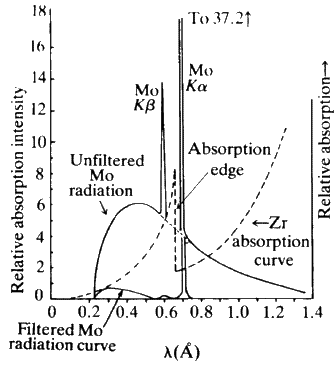
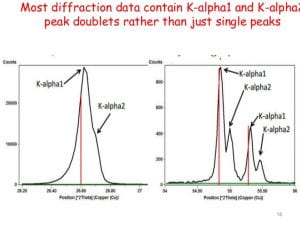
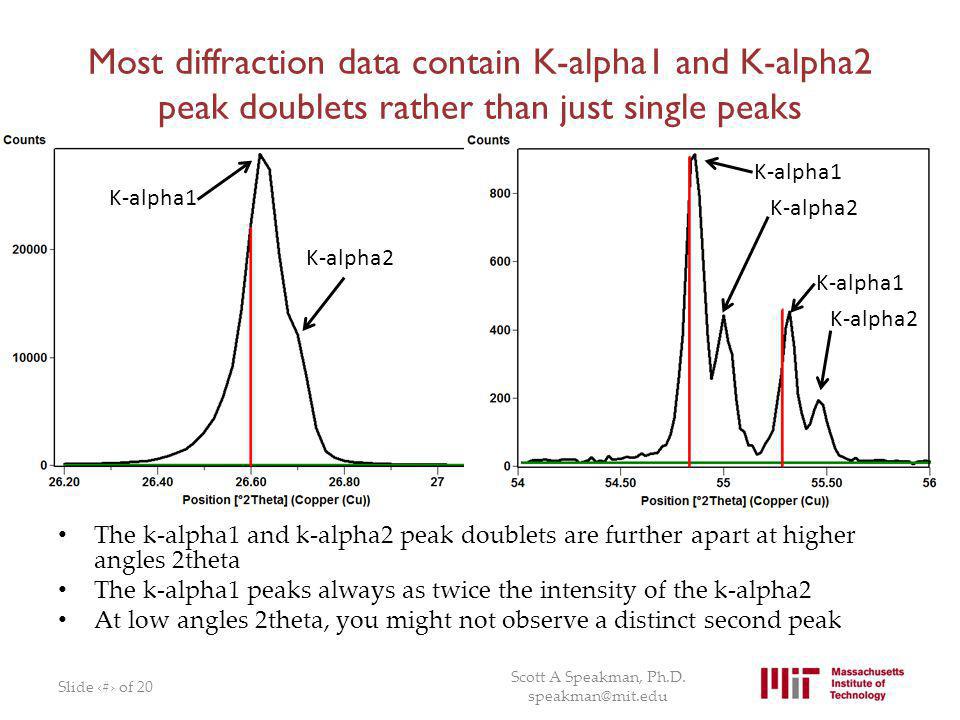
An X-ray tube with a copper target is found to be emitting lines other than those due to copper. The K alpha line of copper is known to have wavelength 1.5405 A

PDF) The K-beta/K-alpha intensity ratios of some elements at different azimuthal scattering angles at 59.54 keV

The K-beta/K-alpha intensity ratios of some elements at different azimuthal scattering angles at 59.54 keV
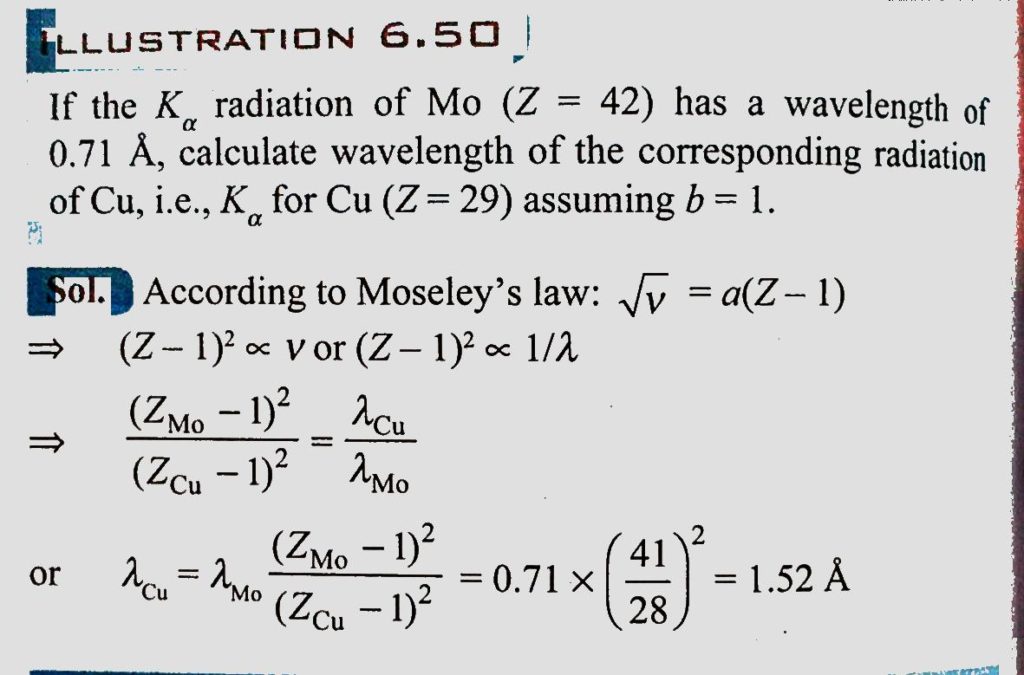
If the K alpha radiation of Mo (Z = 42) has a wavelength of 0.71 A, calculate wavelength of the corresponding radiation of Cu, i.e., K alpha for Cu (Z=29) assuming b = 1. - Sahay LMS
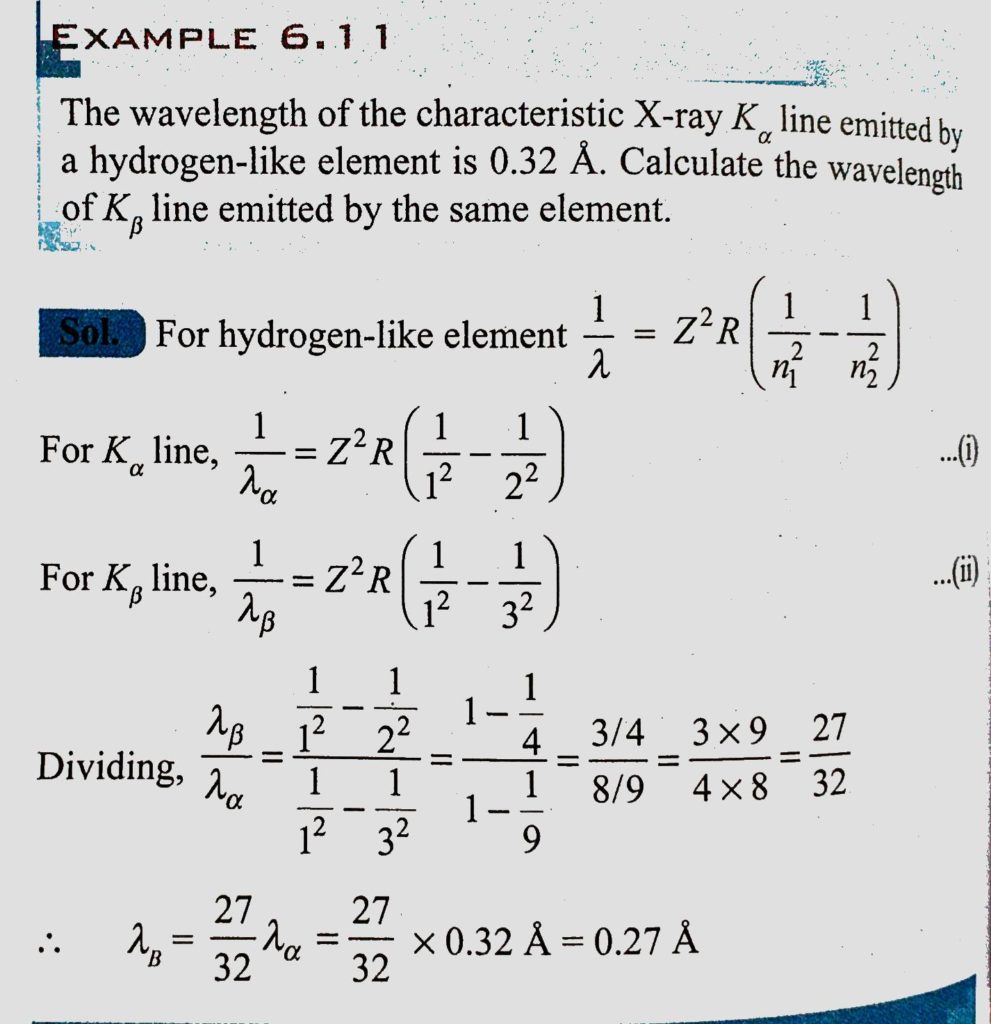
The wavelength of the characteristic X-ray K alpha line emitted by a hydrogen-like element is 0.32 A. Calculate the wavelength of K beta line emitted by the same element. - Sahay LMS
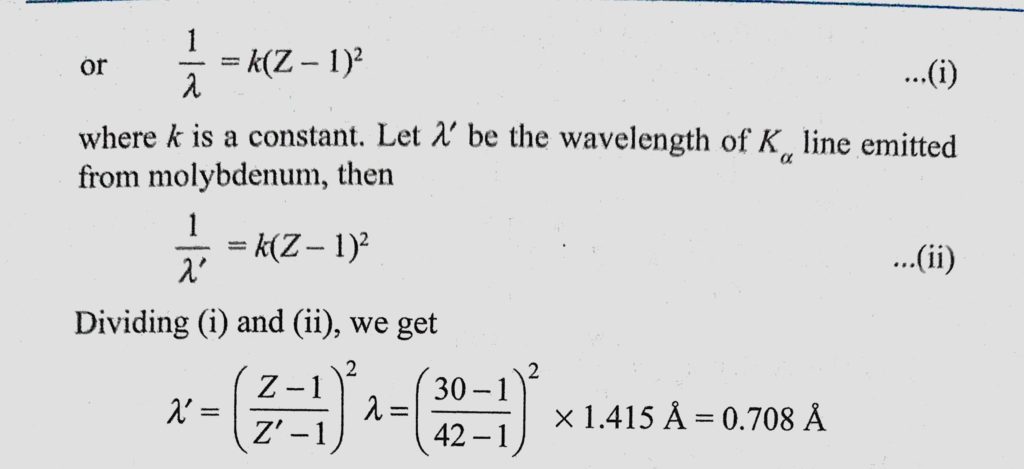


.png)

.jpg)